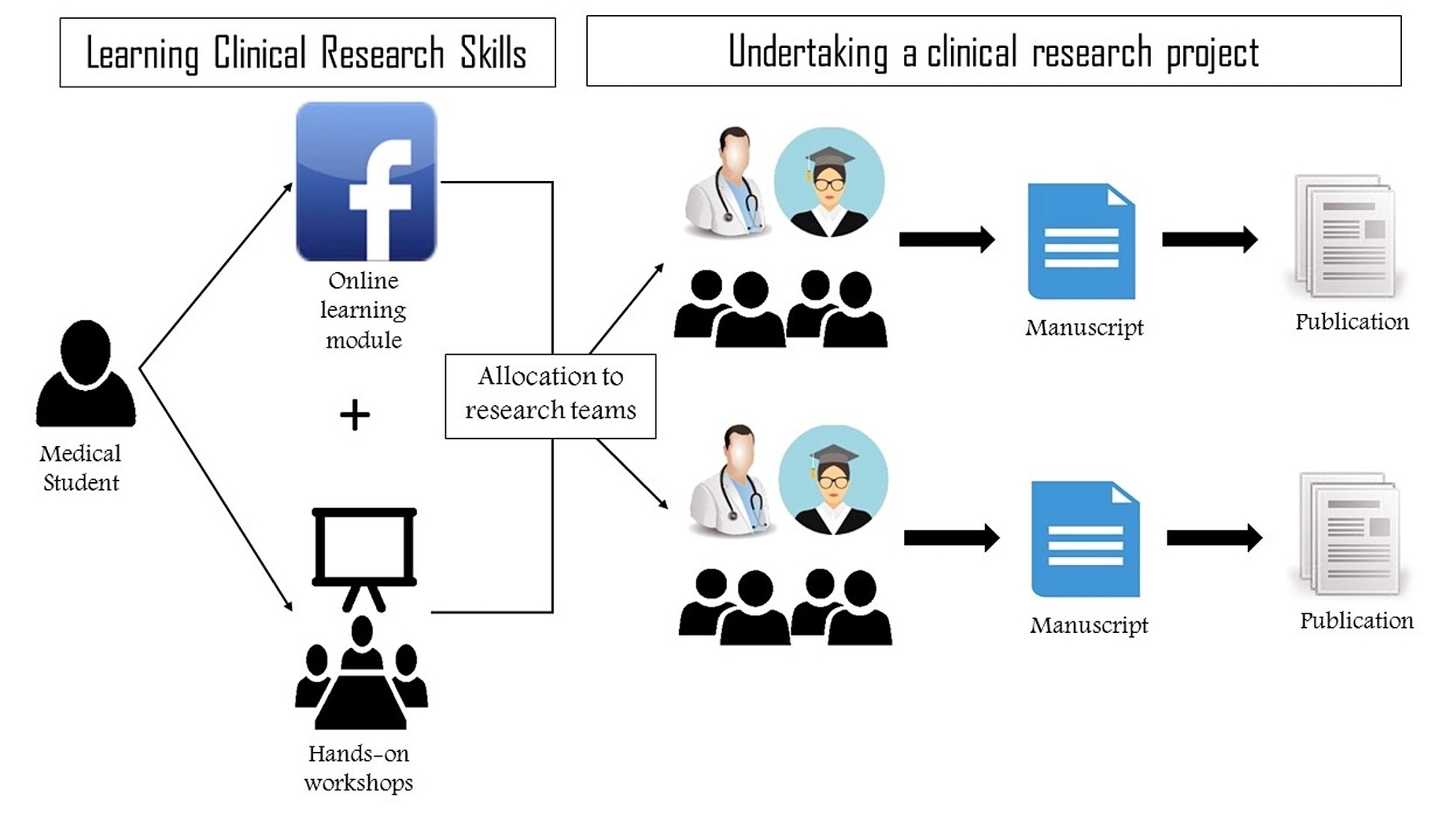
Clinical research refers to studies, or trials, that are done in people. As the developers design the clinical study, they will consider what they want to accomplish for each of the different Clinical Research Phases and begin the Investigational New Drug Process (IND), a process they must go through before clinical research begins.
They are the primary way that researchers find out if a new treatment, like a new drug or diet or medical device (for example, a pacemaker) is safe and effective in people. Often a clinical trial is used to learn if a new treatment is more effective and/or has less harmful side effects than the standard treatment.
When a new drug/device/surgical procedure/treatment or other potential medical innovation is developed it must be thoroughly tested to ensure that it is safe and does what it is supposed to be. This presentation will provide a basic overview of clinical research process.